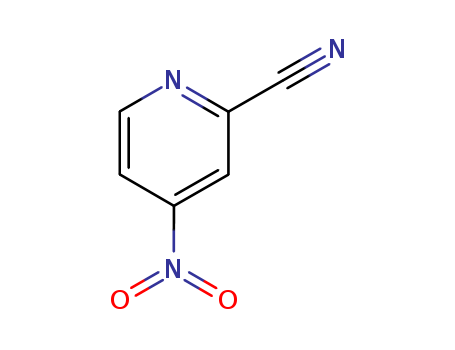
19235-88-2
- Product Name:2-Cyano-4-nitropyridine
- Molecular Formula:C6H3N3O2
- Purity:99%
- Molecular Weight:149.109
Product Details
pd_meltingpoint:72 °C
Purity:99%
Quality Factory Supply 99% Pure 2-Cyano-4-nitropyridine 19235-88-2 with Efficient Delivery
- Molecular Formula:C6H3N3O2
- Molecular Weight:149.109
- Vapor Pressure:0.000979mmHg at 25°C
- Melting Point:72 °C
- Refractive Index:1.583
- Boiling Point:302.629 °C at 760 mmHg
- PKA:-4.23±0.10(Predicted)
- Flash Point:136.825 °C
- PSA:82.50000
- Density:1.418 g/cm3
- LogP:1.38468
2-Cyano-4-nitropyridine(Cas 19235-88-2) Usage
|
General Description |
2-Cyano-4-nitropyridine is a chemical compound with the molecular formula C6H3N3O2. It is a yellow crystalline solid that is used in the production of pharmaceuticals, agrochemicals, and dyes. It is also used as a precursor in the synthesis of various organic compounds. 2-Cyano-4-nitropyridine is known for its strong nitrogen and oxygen atoms, making it useful for creating new chemical compounds with diverse properties. It has been found to exhibit low acute toxicity and is not expected to bioaccumulate in the environment. It is important to handle 2-Cyano-4-nitropyridine with care, as it can be harmful if ingested, inhaled, or comes into contact with skin or eyes. |
InChI:InChI=1/C6H3N3O2/c7-4-5-3-6(9(10)11)1-2-8-5/h1-3H
19235-88-2 Relevant articles
A gas-phase study on the cyclometallation of a series of Cp*Ir(III) complexes bearing bidentate pyrimidine ligands
Becker, Yanik,Huber, Maximilian,Becker, Sabine,Sun, Yu,Niedner-Schatteburg, Gereon,Thiel, Werner R.
, (2021/09/20)
A concerted approach of synthesis and ga...
Cinchona alkaloid amides/dialkylzinc catalyzed enantioselective desymmetrization of aziridines with phosphites
Hayashi, Masashi,Shiomi, Noriyuki,Funahashi, Yasuhiro,Nakamura, Shuichi
supporting information, p. 19366 - 19369 (2013/02/23)
The first highly enantioselective desymm...
Single component N-O chelated arylnickel(II) complexes as ethene polymerisation and CO/ethene copolymerisation catalysts. Examples of ligand induced changes to the reaction pathway
Desjardins, Sylvie Y.,Cavell, Kingsley J.,Hoare, Jason L.,Skelton, Brian W.,Sobolev, Alexander N.,White, Allan H.,Keim, Wilhelm
, p. 163 - 174 (2007/10/03)
Arylnickel(II) phosphine complexes conta...
Antipruritic composition
-
, (2008/06/13)
An antipruritic composition for an oral ...
19235-88-2 Process route
-
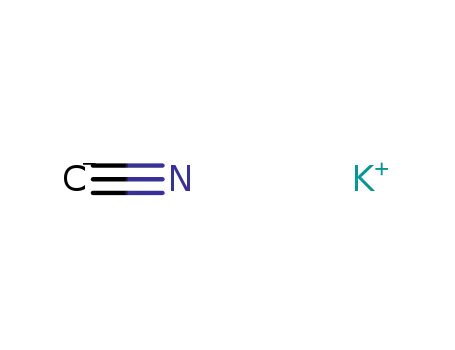
-
potassium cyanide

-
-
C6H7N2O3(1+)*HO4S(1-)

-
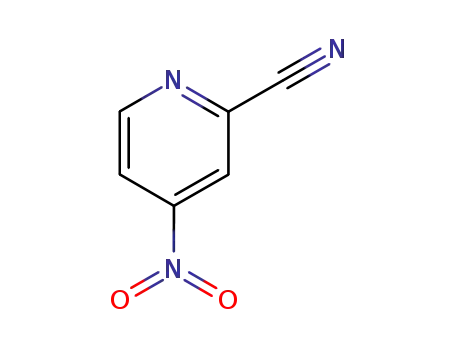
-
19235-88-2
2-cyano-4-nitro-pyridine
| Conditions | Yield |
|---|---|
|
In
water;
at -8 - 20 ℃;
for 18.0833h;
Inert atmosphere;
|
4.15 g |
-
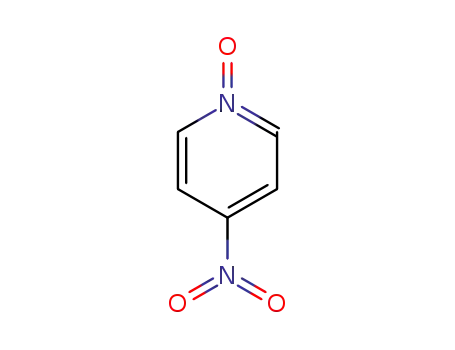
-
1124-33-0
4-nitraminopyridine N-oxide

-
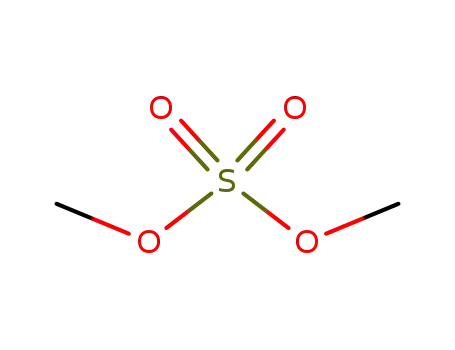
-
77-78-1
dimethyl sulfate

-

-
19235-88-2
2-cyano-4-nitro-pyridine
| Conditions | Yield |
|---|---|
|
In
water;
|
23% |
19235-88-2 Upstream products
-
151-50-8
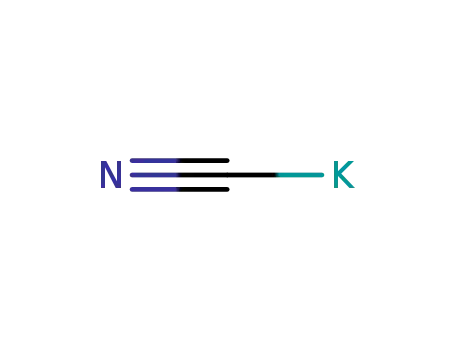
potassium cyanide
-
694-59-7
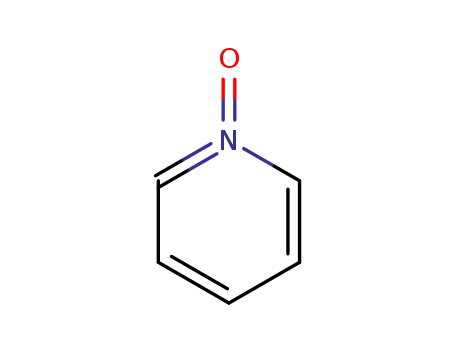
pyridine N-oxide
-
1124-33-0

4-nitraminopyridine N-oxide
-
77-78-1

dimethyl sulfate
19235-88-2 Downstream products
-
62020-02-4
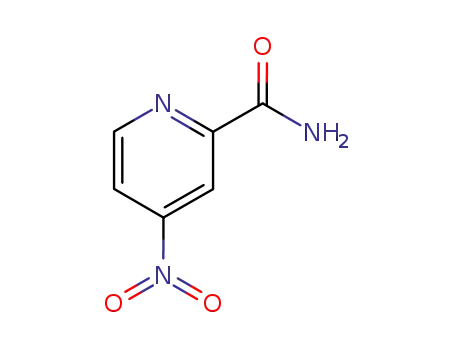
4-nitro-pyridine-2-carboxylic acid amide
-
1426582-46-8
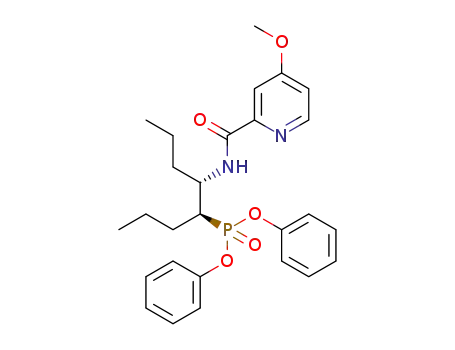
diphenyl (4S,5S)-5-(4-methoxypicolinamido)octan-4-ylphosphonate
-
1414851-99-2
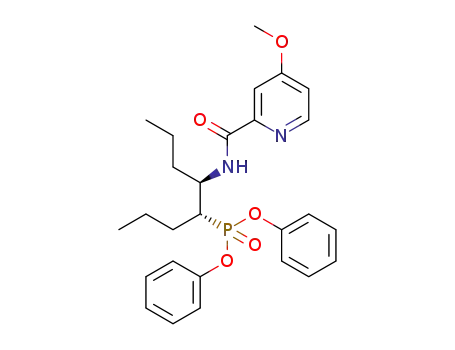
diphenyl (4R,5R)-5-(4-methoxypicolinamido)octan-4-ylphosphonate
-
1426582-59-3
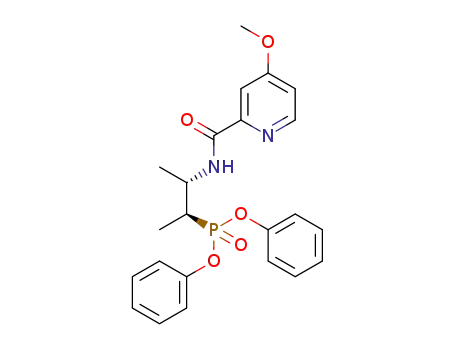
diphenyl (2S,3S)-3-(4-methoxypicolinamido)butan-2-ylphosphonate
Relevant Products
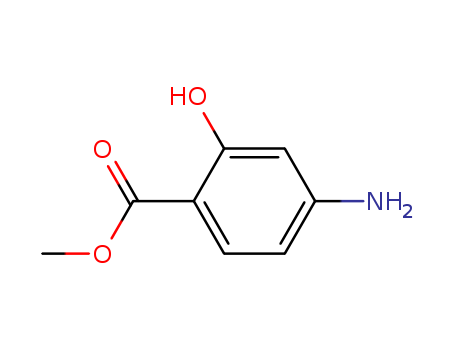
-
Methyl ortho hydroxy para aminobenzoate
CAS:4136-97-4
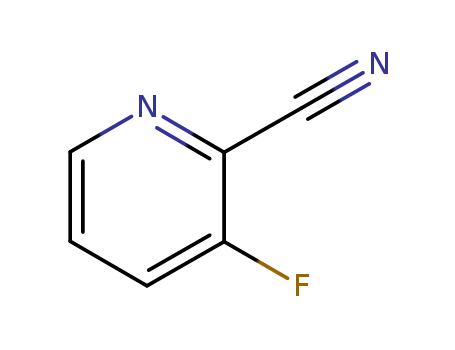
-
2-cyano-3-fluoropyridine
CAS:97509-75-6
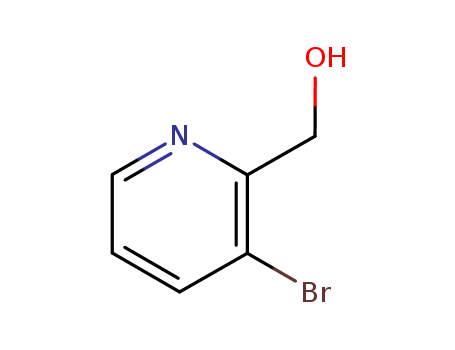
-
2-Hydroxymethyl-3-bromopyridine
CAS:52378-64-0