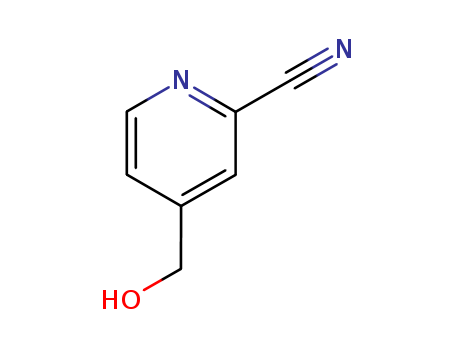
71935-32-5
- Product Name:4-Hydroxymethylpyridine-2-nitrile
- Molecular Formula:C7H6N2O
- Purity:99%
- Molecular Weight:134.137
Product Details
pd_meltingpoint:111-112 °C
Purity:99%
Cosmetics Grade 4-Hydroxymethylpyridine-2-nitrile 71935-32-5 For Sale with Good Price
- Molecular Formula:C7H6N2O
- Molecular Weight:134.137
- Vapor Pressure:0mmHg at 25°C
- Melting Point:111-112 °C
- Refractive Index:1.575
- Boiling Point:340.382 °C at 760 mmHg
- PKA:13.03±0.10(Predicted)
- Flash Point:159.657 °C
- PSA:56.91000
- Density:1.257 g/cm3
- LogP:0.44558
4-(HYDROXYMETHYL)PICOLINITRILE(Cas 71935-32-5) Usage
|
General Description |
4-(Hydroxymethyl)picolinitrile is a chemical compound that consists of a picolinitrile core with a hydroxymethyl group attached to the fourth position. It is commonly used in the pharmaceutical industry as an intermediate in the synthesis of various compounds, including active pharmaceutical ingredients and drug candidates. 4-(HYDROXYMETHYL)PICOLINITRILE is also used in the production of agrochemicals and specialty chemicals. It is an important building block in organic synthesis and is valued for its reactivity and versatility in chemical reactions. 4-(Hydroxymethyl)picolinitrile is a key component in the development of new drugs and has potential applications in a wide range of industries. |
InChI:InChI=1/C7H6N2O/c8-4-7-3-6(5-10)1-2-9-7/h1-3,10H,5H2
71935-32-5 Relevant articles
Di-bromo-Based Small-Molecule Inhibitors of the PD-1/PD-L1 Immune Checkpoint
Konieczny, Magdalena,Musielak, Bogdan,Kocik, Justyna,Skalniak, Lukasz,Sala, Dominik,Czub, Miroslawa,Magiera-Mularz, Katarzyna,Rodriguez, Ismael,Myrcha, Maja,Stec, Malgorzata,Siedlar, Maciej,Holak, Tad A.,Plewka, Jacek
supporting information, p. 11271 - 11285 (2020/11/09)
Immune checkpoint blockade is one of the...
PROBES FOR IMAGING HUNTINGTIN PROTEIN
-
, (2016/03/22)
Provided are imaging agents comprising a...
HETEROCYCLIC COMPOUND AND H1 RECEPTOR ANTAGONIST
-
Paragraph 0271; 0272; 0273, (2013/04/13)
A heterocyclic compound useful as an ant...
A new photolabile protecting group for release of carboxylic acids by visible-light-induced direct and mediated electron transfer
Borak, J. Brian,Falvey, Daniel E.
supporting information; experimental part, p. 3894 - 3899 (2009/10/02)
(Chemical Equation Presented) A new aque...
71935-32-5 Process route
-
![2-cyano-4-[{(tert-butyldimethylsilyl)oxy}methyl]pyridine](/upload/2025/3/98aab3db-a796-41a7-a2c4-ebc00a9624a8.png)
-
117423-43-5
2-cyano-4-[{(tert-butyldimethylsilyl)oxy}methyl]pyridine

-
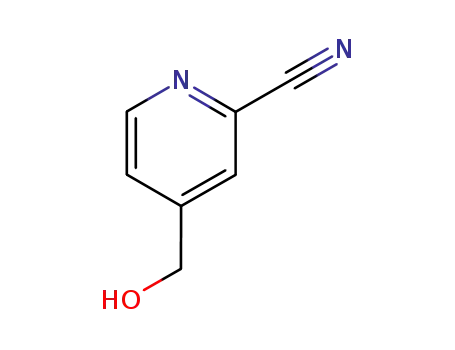
-
71935-32-5
2-Cyano-4-(hydroxymethyl)pyridine
| Conditions | Yield |
|---|---|
|
With
cation exchanger Dowex 50 W*2;
In
methanol;
for 18h;
|
93% |
|
With
sulfuric acid;
In
ethanol;
Reflux;
|
78.8% |
|
With
sulfuric acid;
In
methanol;
at 20 ℃;
for 1h;
|
74% |
|
With
tetrabutyl ammonium fluoride;
In
tetrahydrofuran;
at 20 ℃;
for 2h;
|
73% |
|
With
tetrabutyl ammonium fluoride;
In
tetrahydrofuran;
at 20 ℃;
for 1h;
|
68% |
|
With
Dowex-50W-H+ ion-exchange resin;
In
methanol;
for 18h;
|
4.82 g |
|
In
methanol;
|
-
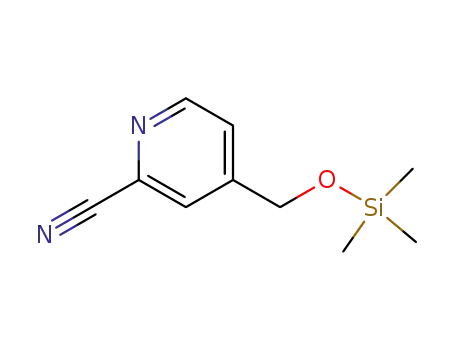
-
754218-90-1
2-cyano-4-(trimethylsilyloxymethyl)pyridine

-

-
71935-32-5
2-Cyano-4-(hydroxymethyl)pyridine
| Conditions | Yield |
|---|---|
|
With
tetrabutyl ammonium fluoride;
In
tetrahydrofuran;
at 20 ℃;
for 3h;
|
32% |
71935-32-5 Upstream products
-
90417-33-7
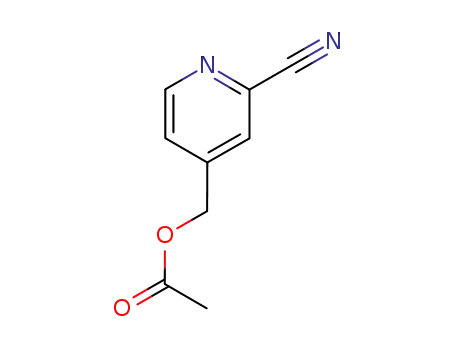
4-Acetoxymethyl-2-cyanopyridine
-
117423-43-5
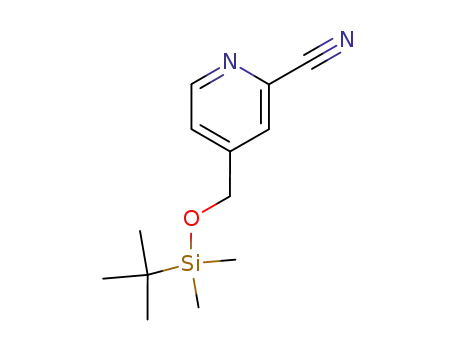
2-cyano-4-[{(tert-butyldimethylsilyl)oxy}methyl]pyridine
-
90005-92-8
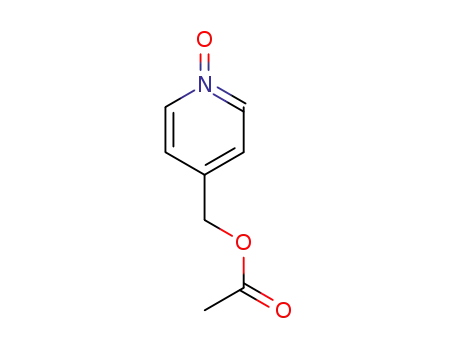
4-(Acetoxymethyl)pyridine N-oxide
-
754218-90-1

2-cyano-4-(trimethylsilyloxymethyl)pyridine
71935-32-5 Downstream products
-
131747-70-1
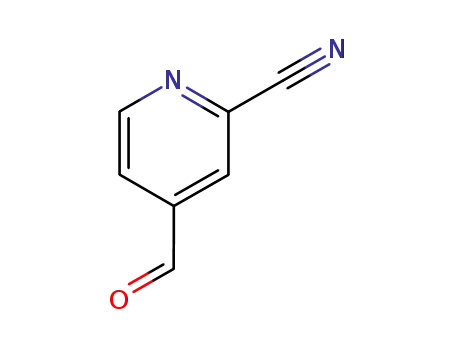
2-Cyano-4-pyridinecarboxaldehyde
-
153993-99-8
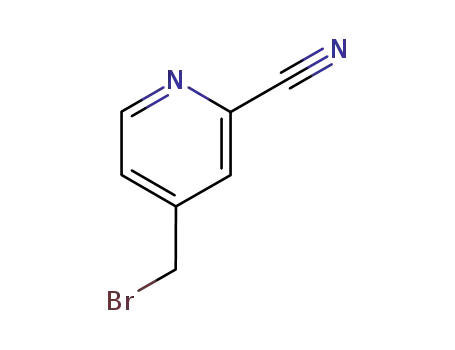
4-(bromomethyl)pyridine-2-carbonitrile
-
59663-96-6
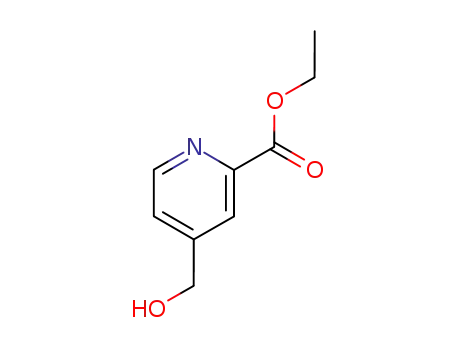
ethyl 4-(hydroxymethyl)-pyridine-2-carboxylate
-
1373423-98-3
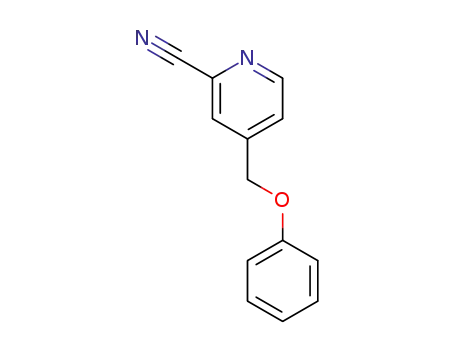
4-[(phenyloxy)methyl]-2-pyridinecarbonitrile
Relevant Products
-
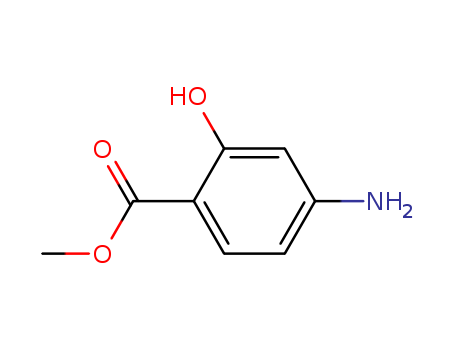
Methyl ortho hydroxy para aminobenzoate
CAS:4136-97-4
-
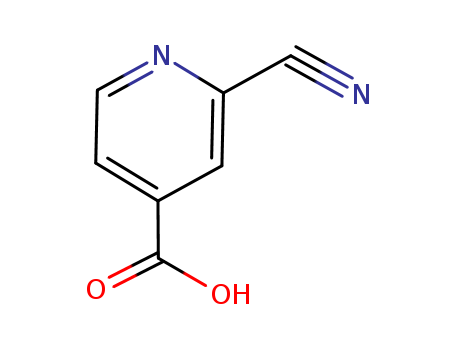
2-cyanoisonicotinic acid
CAS:161233-97-2
-
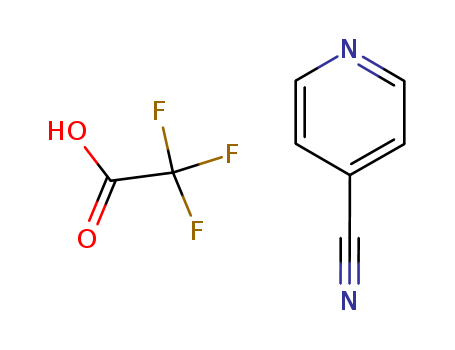
4-cyanopyridine monotrifluoroacetate
CAS:29885-70-9